Medical devices or in vitro diagnostic devices that are placed on the European market, must meet the requirements of their respective Directives or Regulations. Conforming products receive the CE mark label as evidence of compliance.
MARACA International offers a complete list of services to assist the manufacturer in obtaining the CE mark for his products.
Medical devices or in vitro diagnostic devices that are placed on the US market, must meet the requirements of their respective Code of Federal Regulations (CFRs).
MARACA International offers a complete list of services to assist the manufacturer in obtaining FDA clearance or approval for his products.
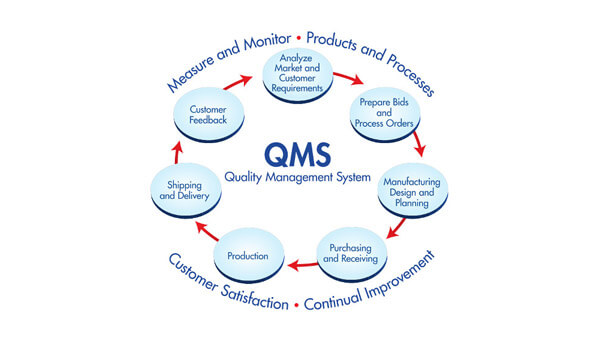
Why implement a quality system? Regulations for Medical Devices and In vitro Diagnostic Devices include quality system requirements.
The European MD and IVD Directives and MD and IVD Regulations include quality systems requirements. EN ISO13485:2012 is the harmonized standard for quality system requirements and ISO13485 certification provides a manufacturer several advantages.
The European Device Directives and Regulations, the US Device regulations and the China Device regulations require risk management as part of product development in the product dossier to authorize your products for distribution in their territories. A good risk analysis and evaluation is central to the development process and will be a valuable tool for troubleshooting and customer service.
To stay competitive in a quickly changing environment, with changing employees, regulations, standards and customer expectations, a company continuously needs training.
MARACA International has established a series of training modules around the new European Medical Device Regulation, the European IVD Regulation, the US Medical Device regulations, the Quality System, ISO13485, ISO14971, design control, risk management and technical documentation and conformity assessment.
Part of performance evaluations in Europe, the USA and China is to search, collect and evaluate the available scientific literature using a systematic search approach. MARACA International can perform such a systematic review of the scientific literature and provide a performance evaluation plan and report. This report will identify the gaps in clinical evidence for your product and guide the development of a clinical study plan when needed.